Outline
On a diagram such as the Txy and Pxy diagrams, the line connecting the boiling point curve and the dew point curve in parallel is called a tie line.
The relationship between the material balance on this line is called the leverage principle for distillation.
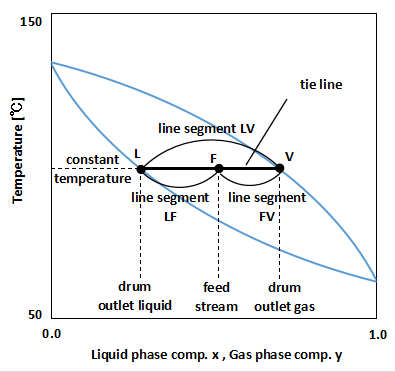
The most easily imagined is the principle of leverage on a Txy diagram. The graph above shows the Txy diagram and tie line.
The relationship in this graph means that the fluid is fed into the drum as shown in the figure below, and what composition is divided into the outlet liquid and the outlet gas.
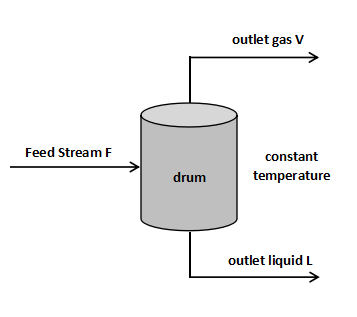
Feeds a fluid whose temperature, pressure, and composition are at point F on the graph.
Point F is between the boiling point curve and the dew point curve and is therefore in a gas-liquid multiphase state.
If the temperature of the drum is kept constant to be the same as the feed temperature, the gas and liquid are separated in the drum and move on the tie line.
As a result, liquid with the composition of point L leaves the bottom of the drum, and gas with the composition of point V leaves the top.
Therefore, the composition of the components after separation can be determined by reading the values of points L and V, which are the intersections of the boiling point curve and dew point curve with the tie line.
Additionally, the material balance can be calculated from the ratio of the lengths of the tie line segments.
$$V=\frac{LF}{LV}F・・・(1)$$
$$L=\frac{FV}{LV}F・・・(2)$$
V is the gas phase molar flow rate, L is the liquid phase molar flow rate, F is the feed molar flow rate.
The gas-phase molar flow rate V and liquid-phase molar flow rate L can be calculated from Eq. (1) and (2).
Of course, if you seriously formulate an equation for the material balance of the entire system and the material balance of each component, you can calculate the same value.
However, the principle of leverage can be used to easily determine the composition and molar amount of each component in a drawing.
For this reason, it is often used in simple studies before using the chemical engineering simulator.
As a chemical engineer, the ability to make precise calculations is of course necessary, but there are times when you are required to quickly calculate rough values, such as at a meeting.
At such times, being able to answer a number quickly by tapping on a calculator will improve your image, so please try to learn it.
In conclusion
The leverage principle for distillation was explained.
This method is useful because it provides a rough idea of the composition after distillation and separation.